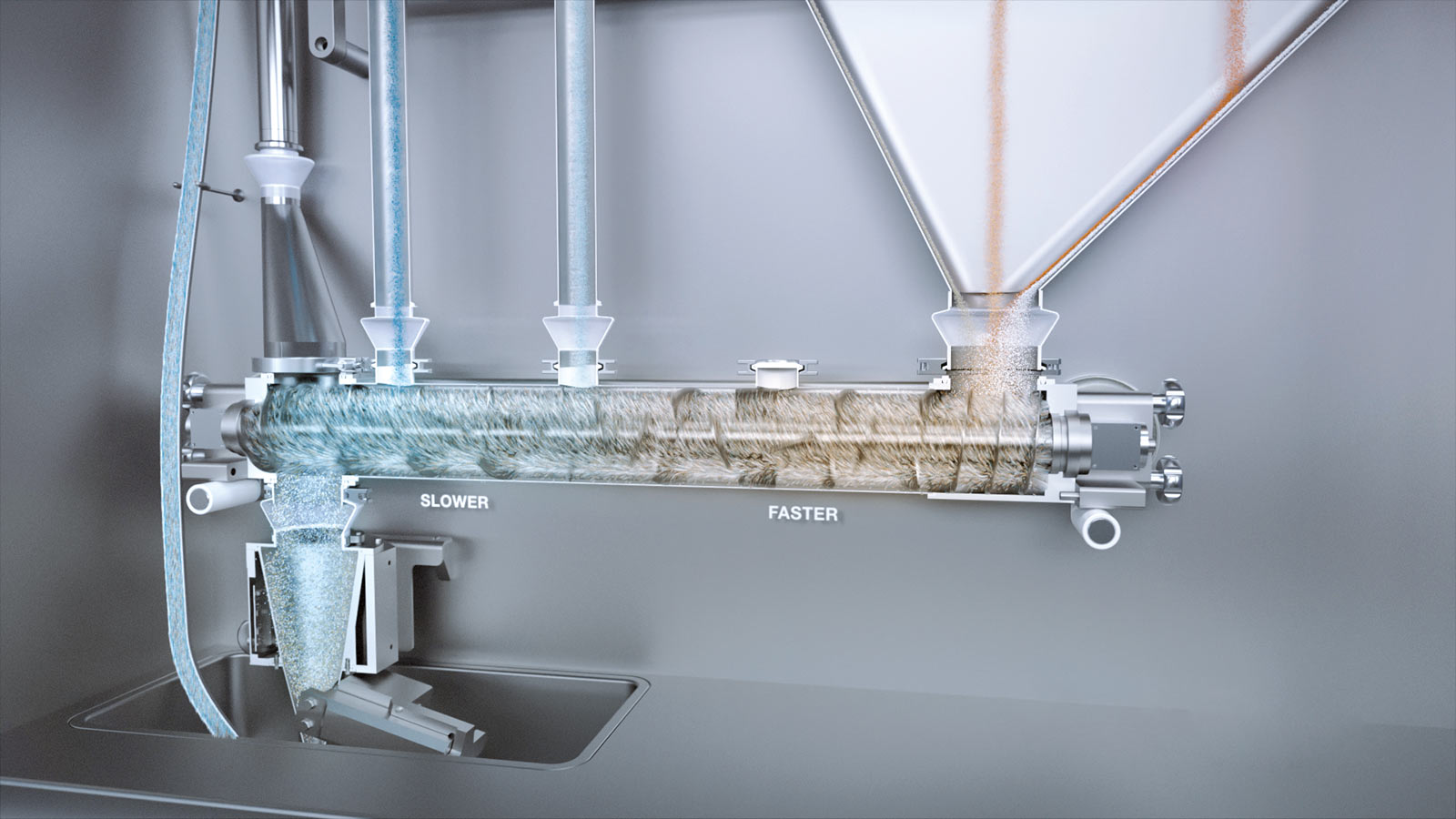
Possibility of mixing with high and low shear.

Continuous Manufacturing could soon become commonplace in the production of drug products and dietary supplements. In recent years, machine manufacturers have been working to bring new technologies to market. At Fette Compacting, too, Continuous Manufacturing is an important area for development, with focus on Direct Compression. This is mainly due to the growing applicability of Direct Compression thanks to advances in particle engineering, which can be combined with lean plant design and efficient process analysis.
Advantages of Continuous Direct Compression
Compared to the batch-to-batch process, Continuous Manufacturing scores with numerous advantages: integrated processes increase efficiency and process reliability, reduced capital investments and operational costs, shorter lead times and flexible batch size, better process control resulting in better product quality, and last but not least shorter time to market for new developed drugs. Finally, Continuous Manufacturing is a quality-driven technology that meets regulatory requirements in the best possible way and is therefore also recommended by the Food and Drug Administration (FDA).
Profile: FE CPS
Compact: one-floor set-up in an existing facility
Modular: maximal flexibility in installation and application
Generic: universal process design for wide range of formulations and throughputs
Safe: dust-tight machine design with separate process and technical areas
Fast: reduced complexity for extremely fast cleaning and changeover
Easy: TRI.EASY for simplified set-up, operation and maintenance with one terminal for all processes
Controlled: inline process monitoring using fully embedded spectroscopic instruments (ePAT)
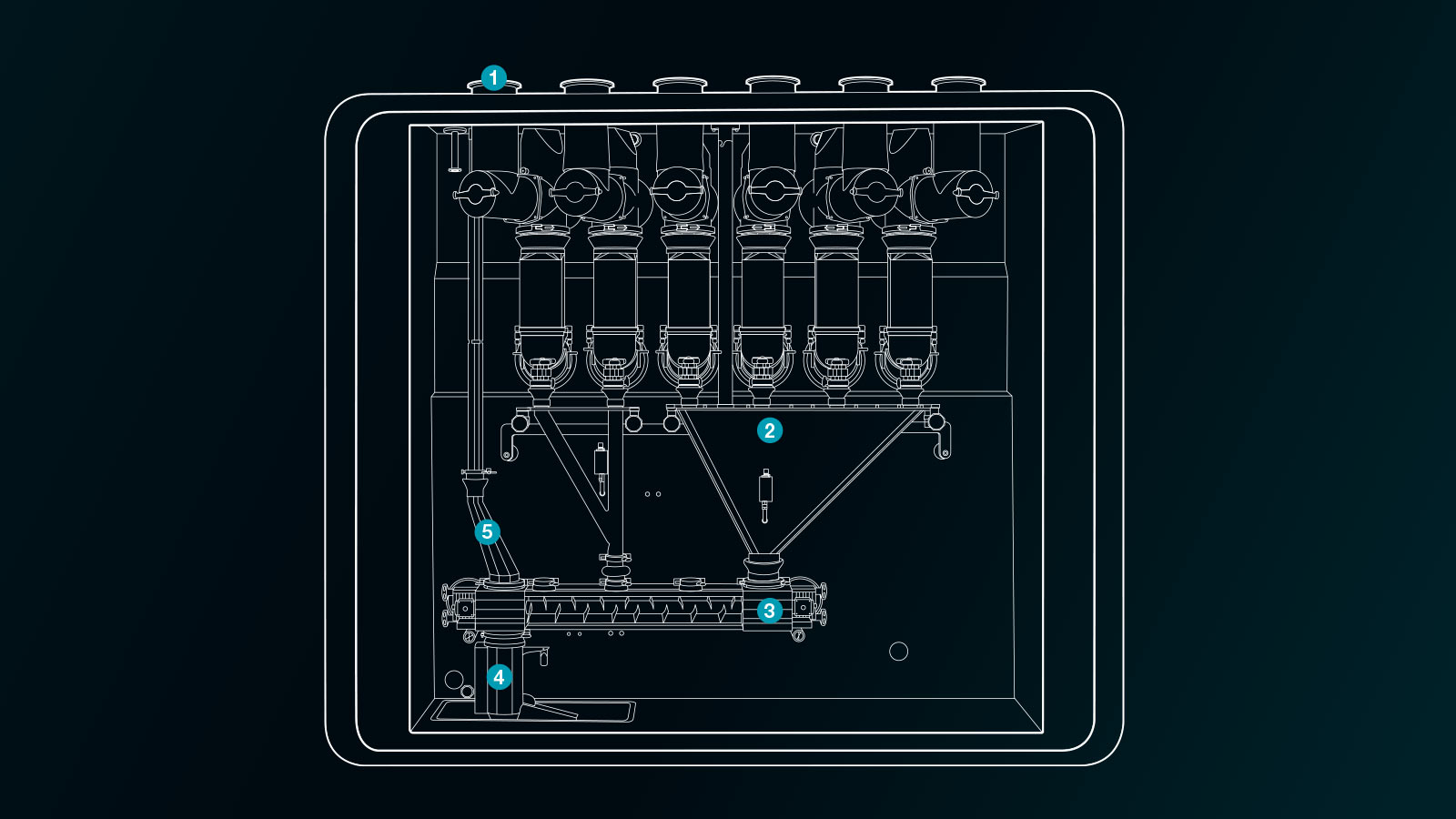
The developers at Fette Compacting have achieved a breakthrough in 2022 in the form of the modular FE CPS dosing-mixing unit. This unit proves to be particularly compact and, in combination with a rotary tablet press and one central operating terminal, forms a complete Continuous Direct Compression line. The entire line can easily be integrated into existing tablet production facilities in a single level set-up. It relies on a dust-tight design as a standard, ease of operation and maintenance as well as fast cleaning and product changeover. The generic machine and process design is based on the same philosophy as for the tablet presses: only a few format parts need to be exchanged to switch over between products. As a result, the FE CPS can efficiently be used in a multi-product production environment.
The path of the powder
A quality-by-design approach was adopted throughout the entire development phase, resulting in the generic process design of the FE CPS. This was complemented by scientific testing of the various individual unit operations using a wide range of different powders and formulations to fully understand the process dynamics. As a result, the FE CPS can process a wide range of ingredients at a wide range of throughputs from 5 to 200 kilograms per hour. Thanks to its generic design the FE CPS is extremely flexible and therefore widely applicable in any powder processing application where various single ingredients need to be accurately dosed, blended and transferred to a downstream process. This can be tablet compression but also dry or wet granulation.
Behind this flexibility is an ingenious machine design of successive unit operations that the powder passes through on its way from the multiple material inlet ports down to the blend outlet port, which is connected to the inlet of the downstream tablet press. The individual unit operations are presented in more detail below.
1. Material feeding
The FE CPS has up to six material infeed ports. A material infeed can be a single ingredient or a pre-mix of multiple ingredients. A pre-mix is used when the total number of ingredients exceeds six or when the concentration of an ingredient in the formulation is particularly low. Each material infeed port is equipped with an ARS (Automatic Refill Systems), which must feed the material reliably and consistently to the next unit operation in an intermittent way. As single ingredients can have extreme powder characteristics in terms of density, flow ability, cohesion, adhesion, etc., accurate and reliable refilling can easily be disturbed by powder sticking, blocking, ratholing and other powder phenomena. As this first unit operation is critical for the correct operation of the entire line, Fette Compacting has developed its own refilling system with special screws. They can convey very challenging raw material into the dosing process in a reliable and consistent manner.
2. Dosing
Dosing is at the heart of the FE CPS. Up to six gravimetric (Loss-in-Weight, LIW) powder dosing feeders are used here. For each feeder, the concentration of the respective material in the formulation is stored in the product recipe. Combined with the required line throughput, the control system automatically calculates the required feed rate. The LIW feeders use twin screws to dose the material at the required feed rate and with minimal feed variability into the next unit operation. Different types of twin screws are available for optimal LIW dosing performance for any powder at any feed rate.
3. Blending
The FE CPS is equipped with a Fette Compacting proprietary purpose-designed horizontal tubular continuous powder blender. Its ingenious design differentiates from any existing horizontal tubular blender by its two successive but independent mixing zones, without a dead zone in between. This allows mixing processes with high and low shear intensity to be combined in a single mixer and the best mixing results to be achieved according to formulation requirements. The blender is equipped with four inlet ports: two in the first blender zone and two in the second zone. The various ingredients are fed from the LIW feeders to the inlet ports of the blender using a combination of drop tubes and transfer funnels. Each specific funnel configuration determines which feeder outlet is connected to which blender inlet. Various funnel configurations are available, offering great flexibility to achieve optimal process set-up for a wide range of formulations.
4. In-line quality control
At the outlet of the blender, there is the option of using embedded Process Analytical Technology (ePAT). A near-infrared spectroscopic sensor checks the homogeneity of the mixed powder and concentration of the API, among other things. As a result, process parameters can be quickly adjusted in the event of quality deviations. Especially in product development, it is relevant to continuously record and optimize the mixing process.
5. Conveying
As the last step before tableting, the powder blend must be conveyed to the inlet of the tablet press in a uniform manner without any risk for segregation. This could be done by gravity if the FE CPS is installed above the tablet press but is to be avoided because this would require extremely tall production rooms, while additionally long powder drop heights might induce segregation. For this purpose, a special powder transport system was developed, which conveys the product in dense phase without risk of segregation over a distance of ten meters and more. Via a conveying hose, it reaches the conveyor arm of the FE CPS, which can be flexibly aligned with the inlet of the tablet press. This ensures that the thoroughly blended powder reaches the tablet press without any disturbances and is pressed there in the usual quality. The blend conveyor system also allows for a two-room set up of the direct compression line. The FE CPS can be installed in one room, while the tablet press is installed in the adjacent room. This can be of particular interest in case the system needs to be installed in an existing tablet production facility.
Join the flow
Following the construction of two prototypes, the FE CPS was presented to the trade public for the first time during the Continuous Manufacturing Circle in the summer of 2022. The reactions during the launch event and the many individual machine demonstrations afterwards prove the strong interest of pharmaceutical and nutraceutical manufacturers in this new compact, generic and easy-to-use technology. Multiple product trials with customers have been conducted in the meantime – all with good to excellent results in terms of ease of line set-up, product quality and throughput capacity. It was proven that formulations developed for batch manufacturing could be run on the Continuous Direct Compression line without any change of ingredients. This was even the case for several formulations, which are originally wet and dry granulation based! It proves the huge potential of Continuous Manufacturing, and more particularly of Continuous Direct Compression. Numerous discussions are already taking place with various customers for specific applications.
If you would like to learn more about the FE CPS, simply contact us at tablet@fette-compacting.com.
Our experts will give you more detailed insights and advise you on your individual Continuous Manufacturing project. Two Continuous Direct Compression lines are available for live demonstrations at the sites in Schwarzenbek (Germany) or Mechelen (Belgium). Please feel free to make an appointment and come and see for yourself!