[Translate to English:] In der Smart Factory produziert Boehringer Ingelheim seine Neueinführungen von Arzneimitteln für den Weltmarkt.

Networked production, smaller batches, increased flexibility: with the new Smart Factory, Boehringer Ingelheim aims to manufacture its new drug approvals to the highest efficiency standards. The pharmaceutical specialist has invested around 90 million euros in the Solids Launch (SOL) factory at its Ingelheim site. The plant technology is already highly digitally networked, and these networks are being extended further to enable all processes to control themselves. This will enable the factory to respond independently to changes based on production data, quality parameters, and environmental conditions. “We can now bring new drugs to market even faster, which also benefits patients,” explains Martin Döhms, Process Specialist in the Engineering division at Boehringer Ingelheim.
Automation meets containment
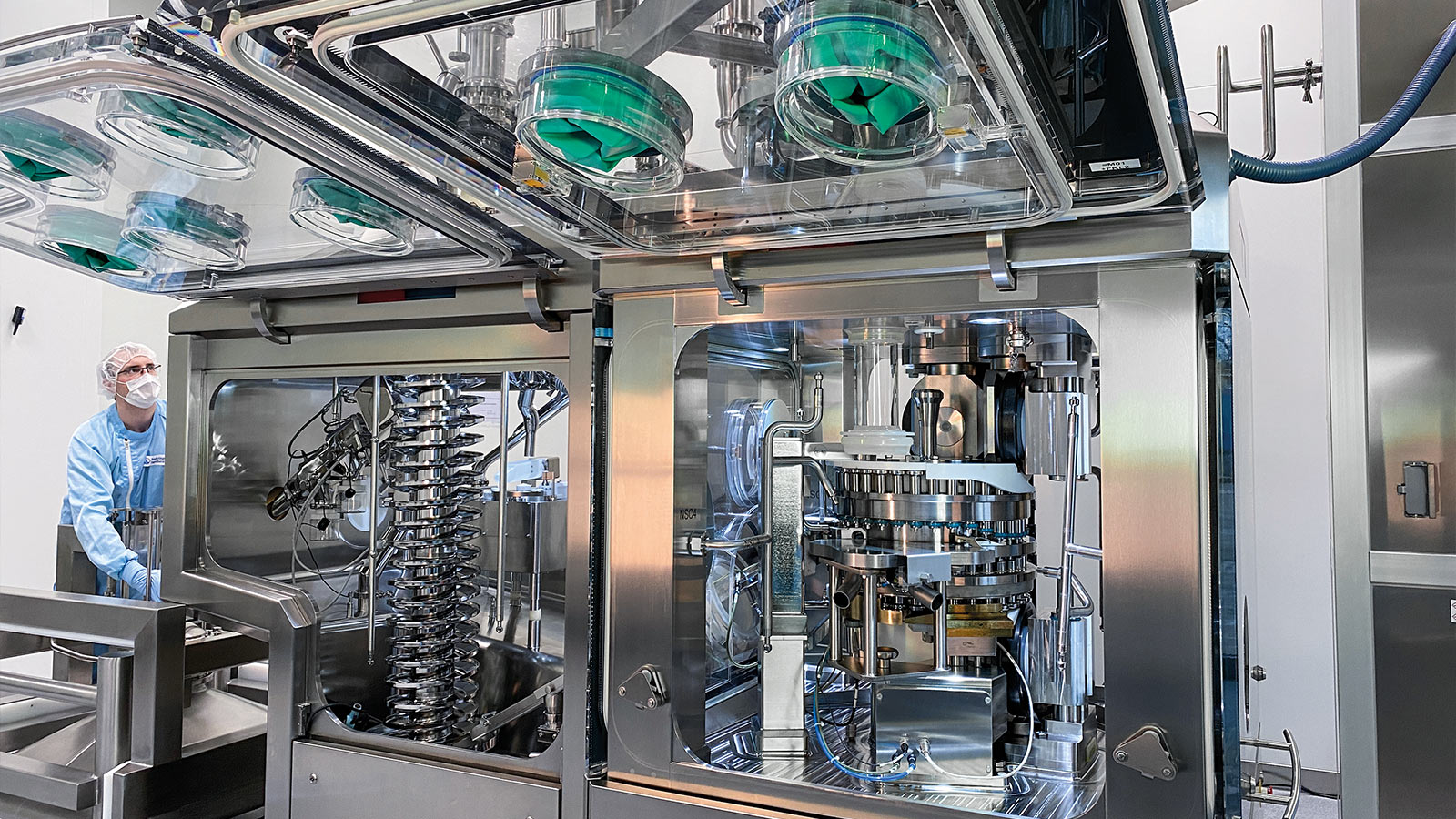
Only medications in tablet form are produced in the SOL, with operator protection featuring containment playing a central role. “For all our automation, the know-how of our well-trained employees continues to be in demand,” claims Martin Döhms. “Around 50 employees work in the new factory, monitoring processes, re-tooling equipment, and continuing to drive forward the industrialization of processes and equipment, among many other tasks. We need suitable containment protection for the operators at the machines. After all, we process active ingredients there up to OEB level 4, i.e., into the highly-active range.”
To process these active ingredients safely and quickly, Boehringer Ingelheim opted for a 2090i WiP (Wash-in-Place) tablet press with isolator and air management from Fette Compacting. The system combines proven technology with maximum flexibility: it has a modular design with the result that many components can be easily replaced. In this way, the tablet press can be quickly converted to other formats. The process equipment is integrated into the isolator, which – like the tablet press – has glove ports and a dual-flap system (Rapid Transfer Port (RTP)) for feeding and discharging materials. This spares operators the strains associated with working while wearing full protective suits. Automatic cleaning of the WiP system provides additional relief and reduces downtime.

The second tablet press used by Boehringer Ingelheim in the factory is an FE55. The high-performance rotary press is suitable for a wide range of products and, with a maximum output of more than 600,000 tablets per hour, delivers particularly high performance. Its innovative filling system enables simple and safe feeding of complex product mixtures.